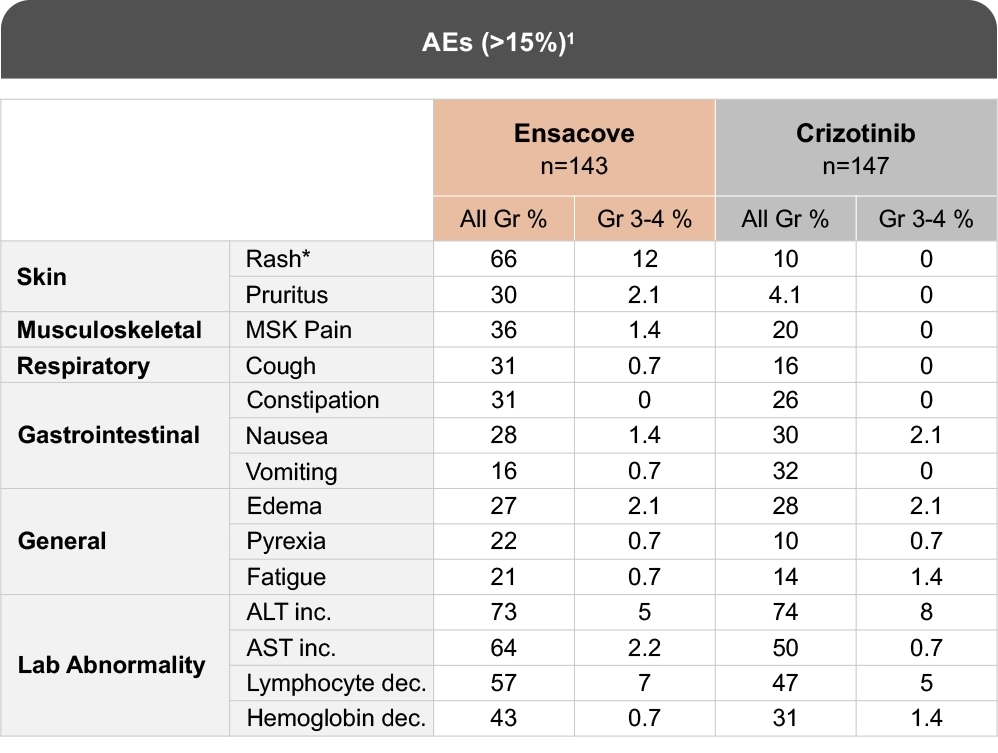
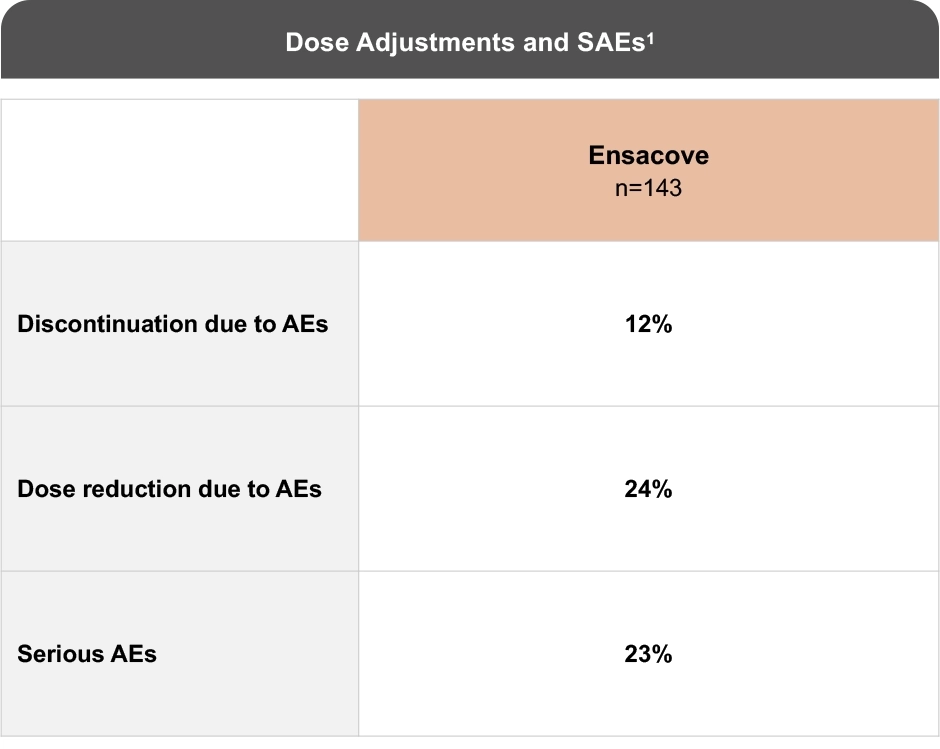
Established Safety Profile Over mFU of 74 Months1
Most common adverse reactions in eXALT3.1
Discontinuation, dose reduction, and serious adverse events.1
- Permanent discontinuation of ENSACOVE due to an adverse reaction occurred in 12% of patients1
- Adverse reactions which resulted in permanent discontinuation of ENSACOVE (≥1%) included increased ALT (2.1%), increased AST (2.1%), pneumonitis/ILD (2.1%), increased blood bilirubin (1.4%), and increased conjugated bilirubin (1.4%)1