Efficacy Proven in First-line Locally Advanced or Metastatic ALK+ NSCLC in Patients Who Have Not Previously Received an ALK-Inhibitor1
Efficacy demonstrated in eXalt3.
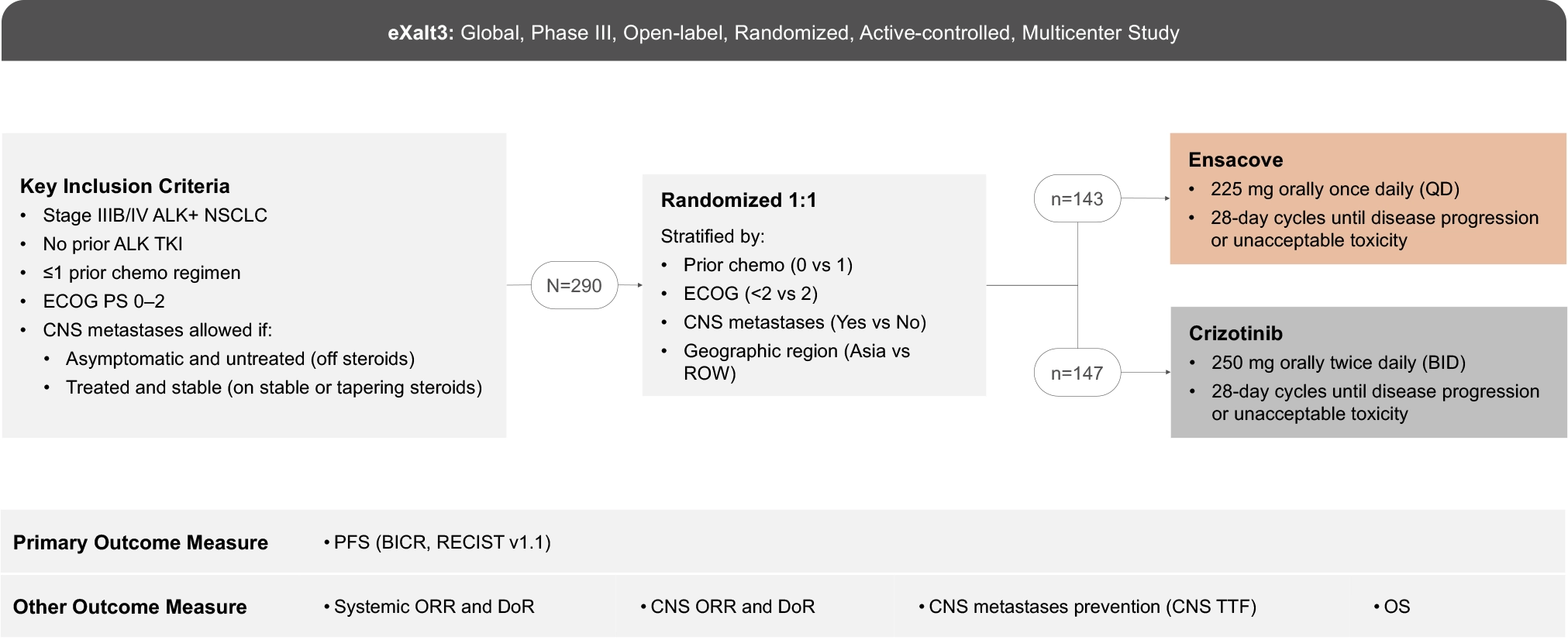
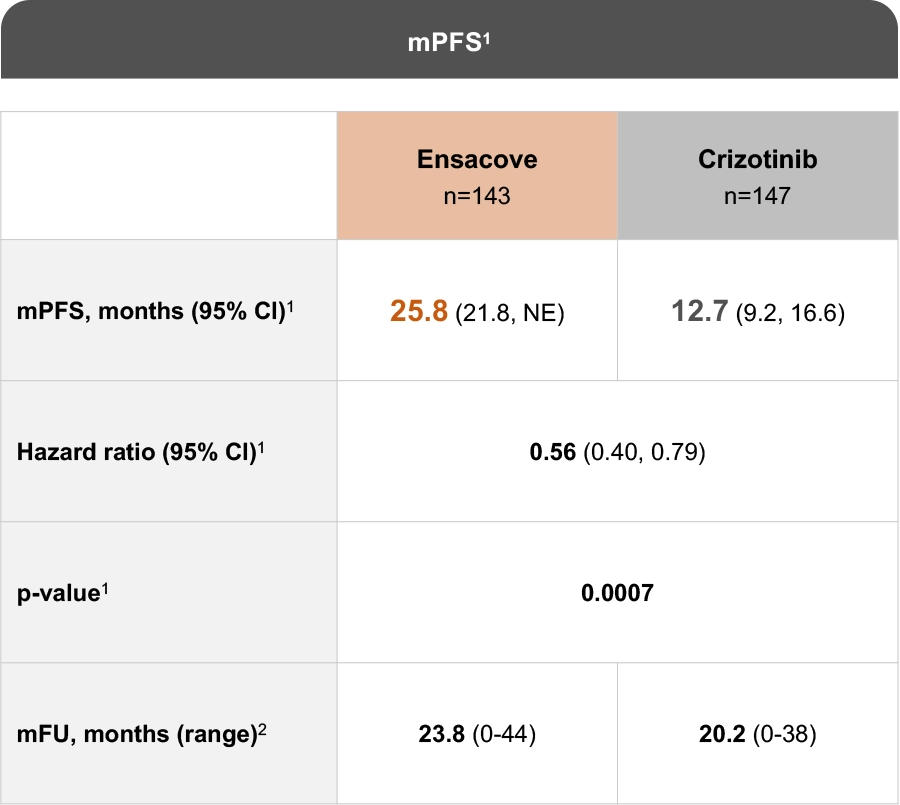
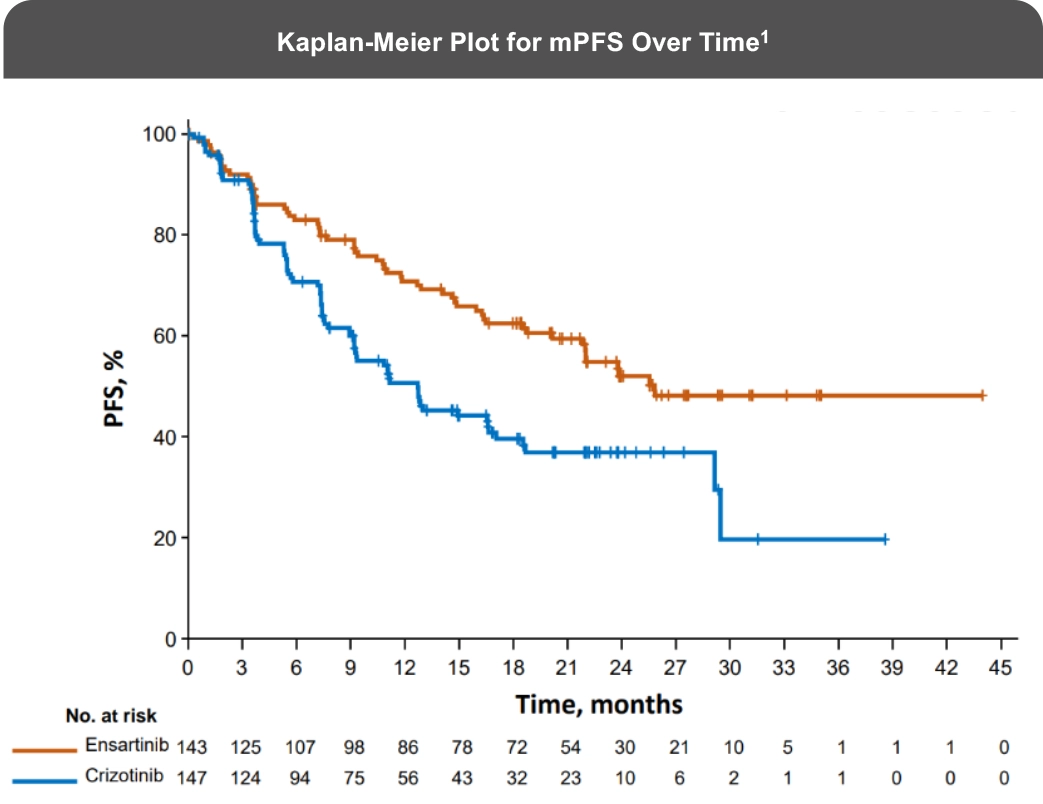
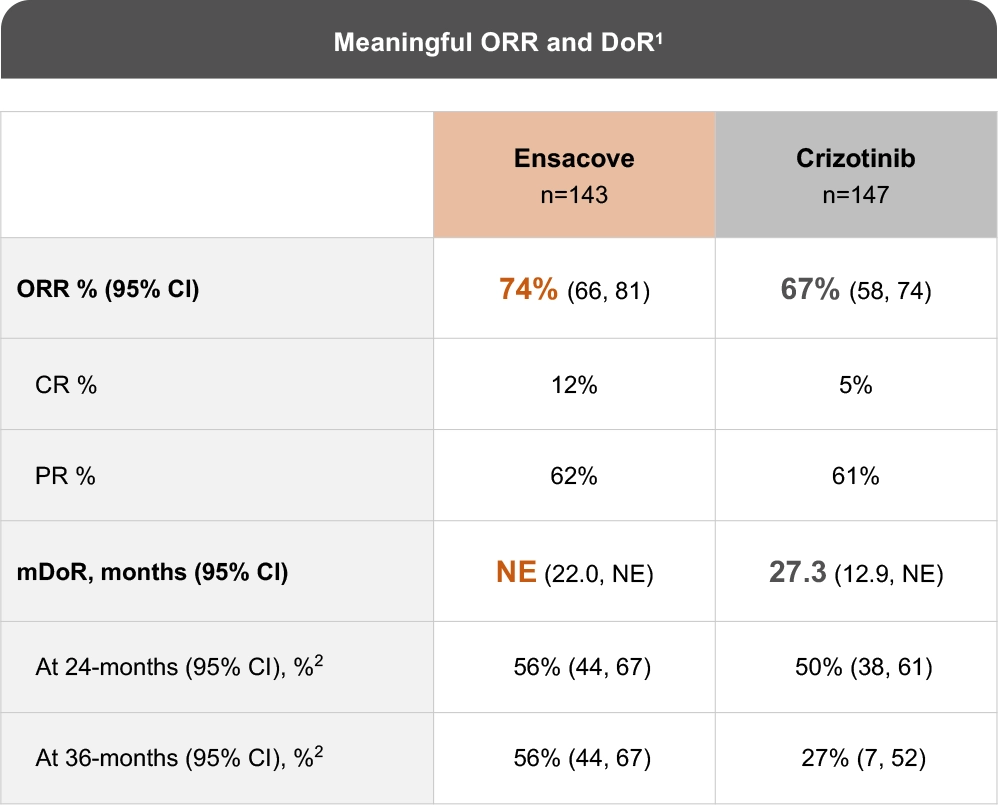
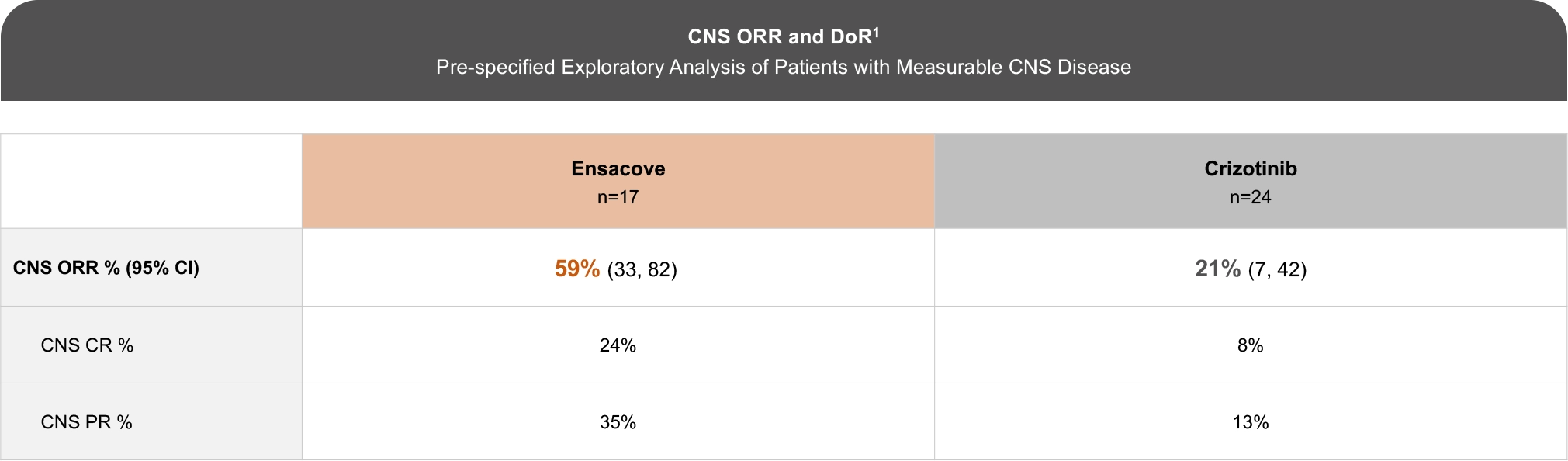
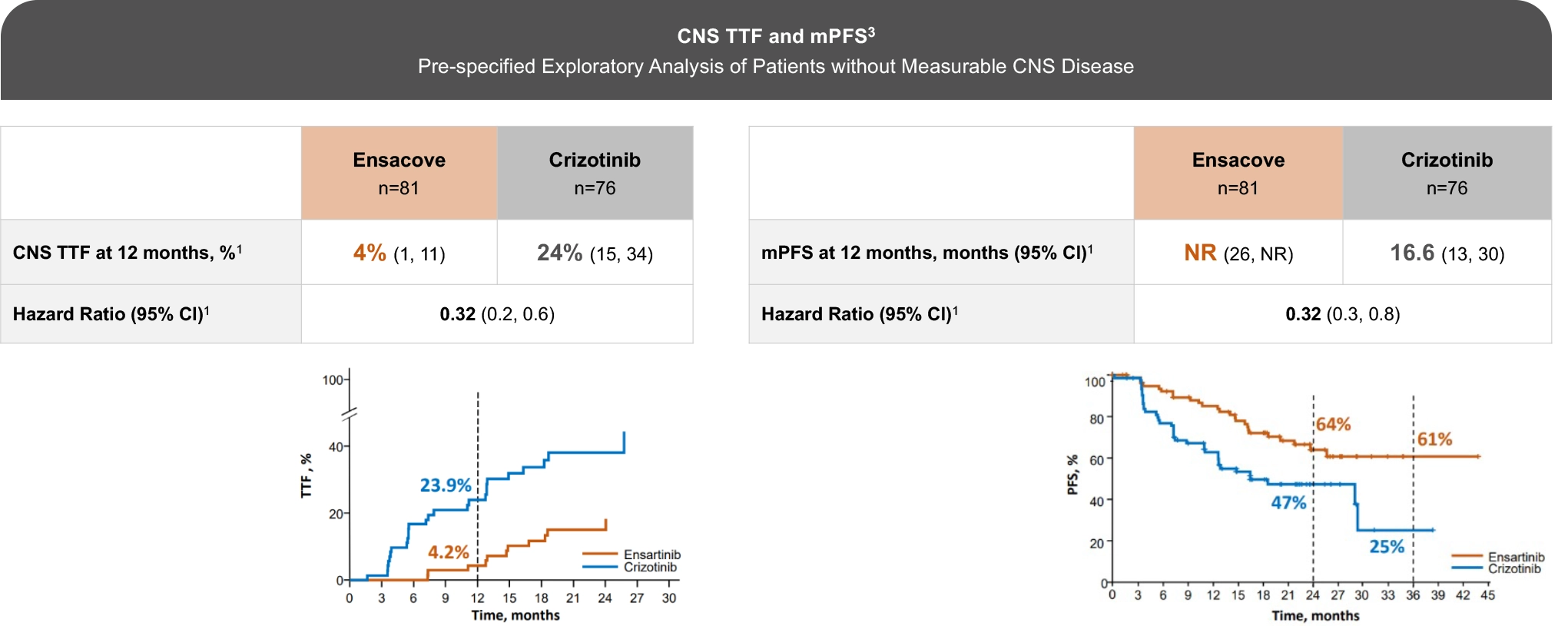
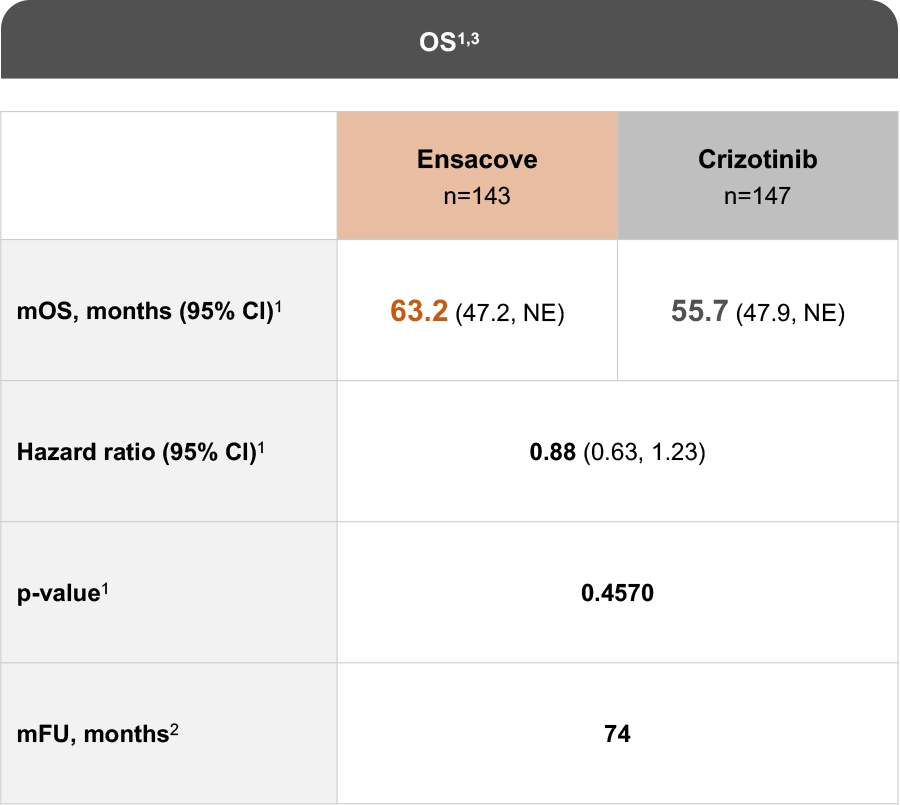
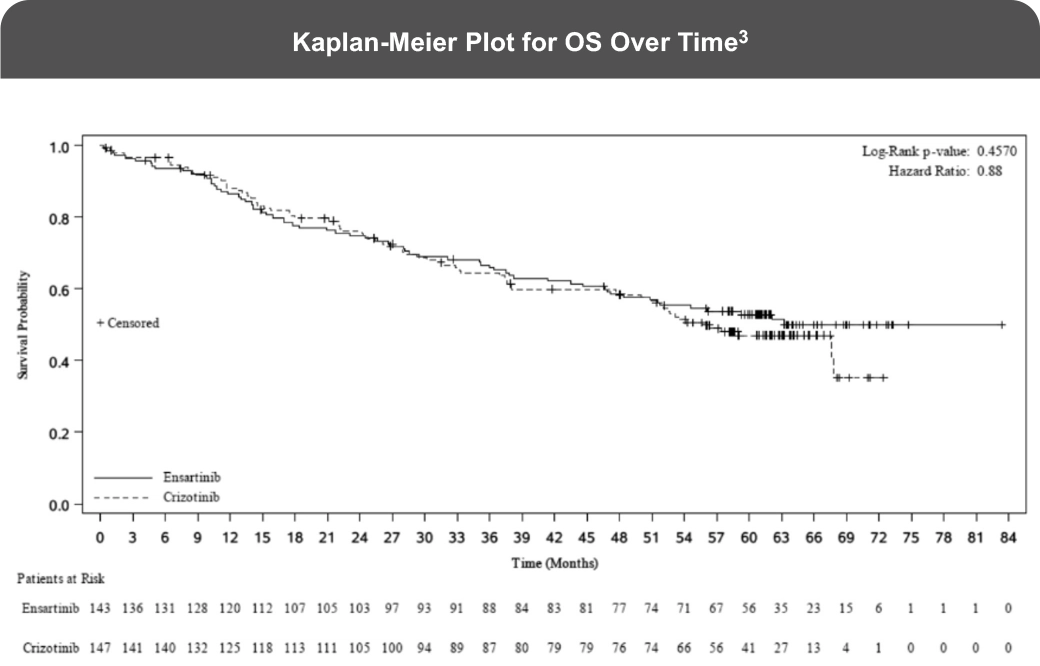
The eXalt3 study evaluated efficacy and safety in patients with ALK+ locally advanced or metastatic NSCLC who had not previously received an ALK TKI.1